Parkinson’s disease (PD) and Dementia with Lewy Bodies (DLB) impact over 2 million people in the United States.
The goal of the lab is to determine how pathologic α-synuclein, and genes implicated in Parkinson’s disease cause neuron dysfunction, leading to devastating symptoms including impaired movement, cognitive dysfunction, and psychiatric symptoms. We are utilizing pharmacological and physiologic strategies to attempt to prevent or rescue defects in neuron function.
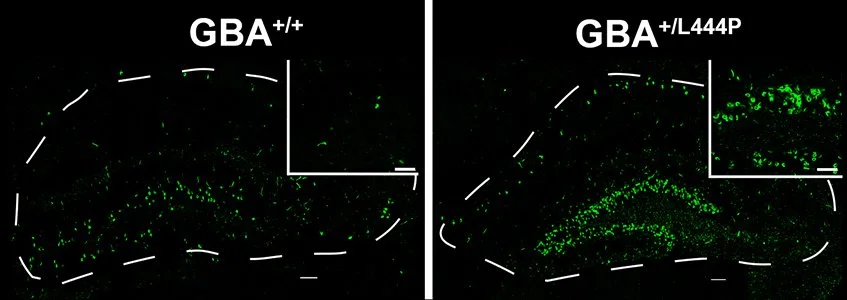
How do mutations in GBA1 and glycosphingolipids affect cognition? Heterozygosity for severe GBA1 mutations confers a 5X increase in risk of cognitive decline in PD. Pathologic α-synuclein is increased in the hippocampus, a brain region important for cognition, in mice heterozygous for GBA1L444P. Do increased levels of the lipid, glucosylsphingosine, caused by GBA1L444P play a role cognitive decline? We are testing novel pharmacologic compounds to reduce pathologic accumulation of glycosphingolipids to prevent cognitive decline in PD and Dementia with Lewy Bodies.
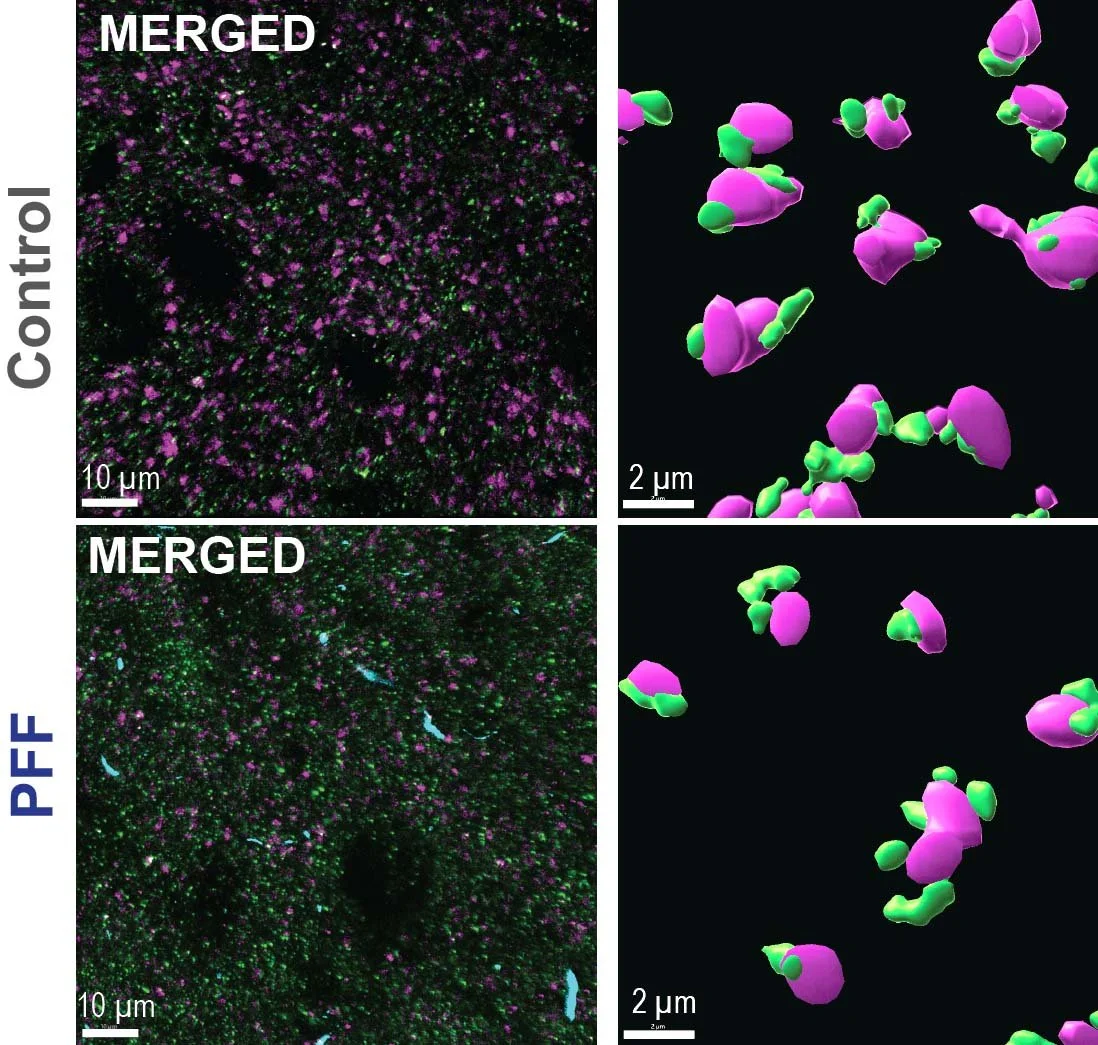
How does early formation of abnormal α-synuclein aggregates impact synaptic structure and function? We want to know if early changes in synaptic function and morphology contributes to cognitive symptoms. We show that α-synuclein aggregates cause robust and reproducible structural changes in synapses. We are also working with collaborators using electrophysiology and fiber photometry to determine how α-synuclein aggregates disrupt synaptic function, and dynamic release of neurotransmitters. Our goal is to find mechanisms to prevent or rescue synaptic defects.